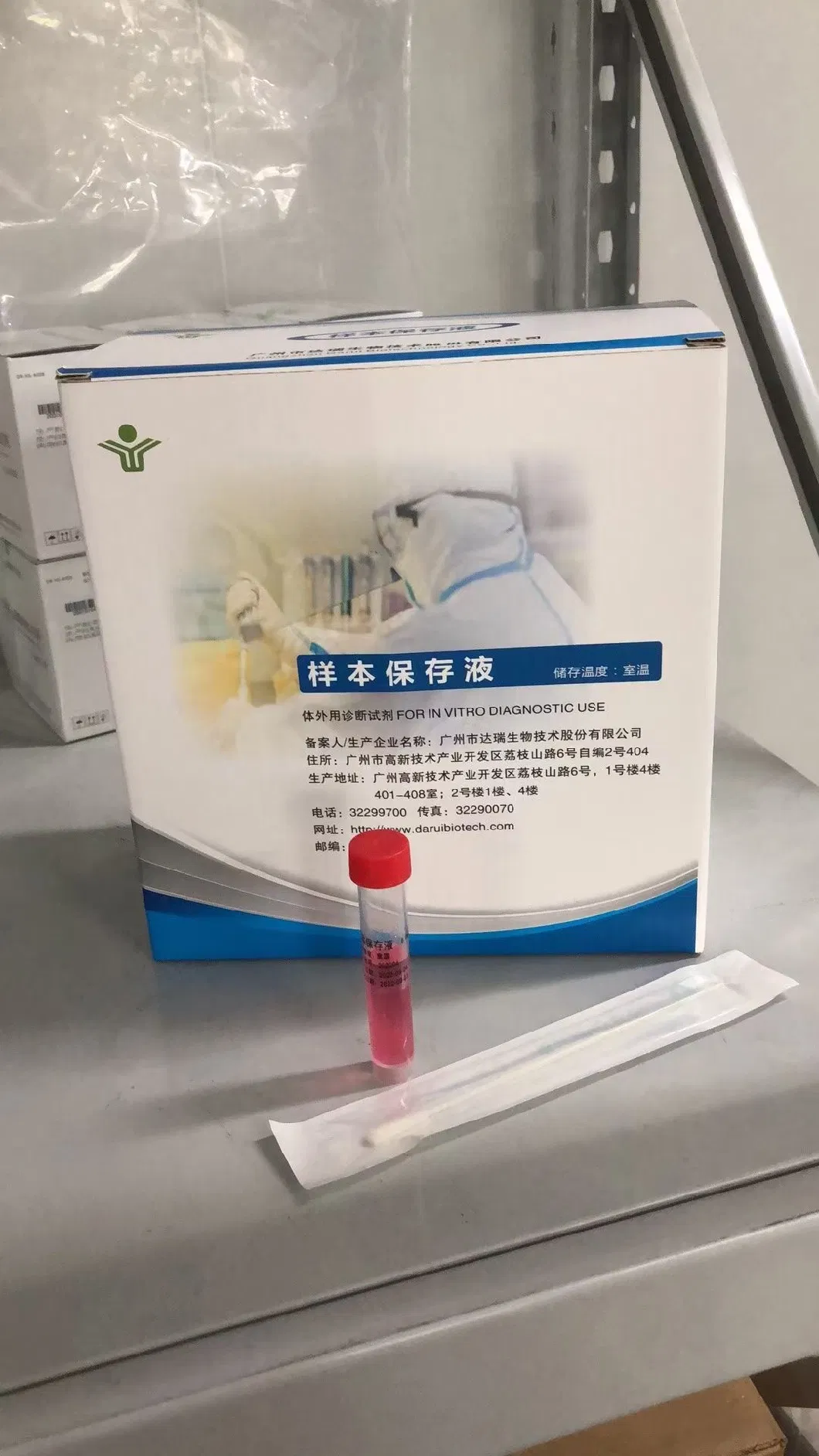
vtm vtm-n transport vials viral media kit with 2 test flock nasopharyngeal throat swab
This product provides a safer condition for collecting, transferring and preserving sample. Guanidine salt and RNA/DNA protectant are added into the solution, making the virus inactive and protecting released RNA/DNA from degradation by RNase or DNase. But it doesn't affect the integrity of the viral nucleic acid.
Convenience: Storage and transportation can be conducted under room temperature.
Preservation: Keep viral nucleic acid from degradation within 7 days at 2~8ºC.
Order Information
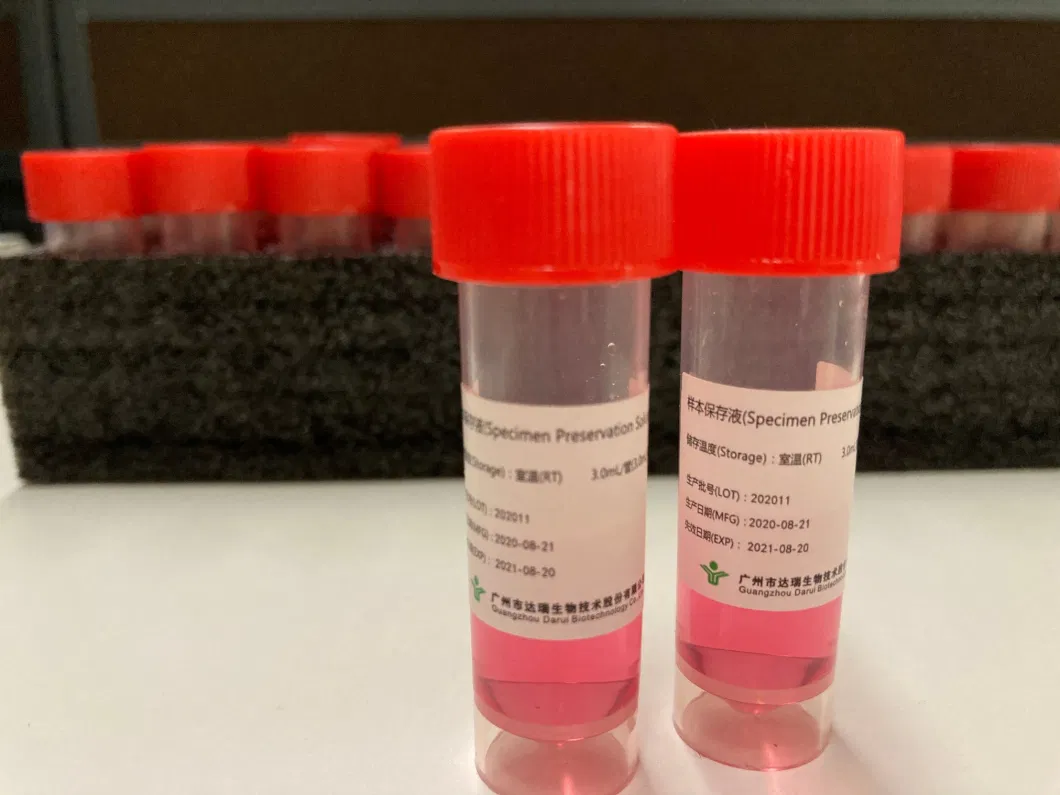
Packing
This product provides a safer condition for collecting, transferring and preserving sample. Guanidine salt and RNA/DNA protectant are added into the solution, making the virus inactive and protecting released RNA/DNA from degradation by RNase or DNase. But it doesn't affect the integrity of the viral nucleic acid.
Convenience: Storage and transportation can be conducted under room temperature.
Preservation: Keep viral nucleic acid from degradation within 7 days at 2~8ºC.
Order Information
| P/N | Description | Package |
| DR-SPS-E006 | 12ml tube with 6ml SPS | 100T/BOX, 1000T/CTN |
Packing
1.0mL/tube, 2.0mL/tube, 3.0mL/tube, 500mL/bottle,
1L/bottle
Intended Use
This product is used to store samples containing
infectious virus, protect DNA or RNA in
samples for subsequent detections, while
eliminating the infectivity of pathogens for
biosafety. This product is convenient to use and
could be widely utilized in hospitals and research
institutes.
Product Component
Tris-HCl, Guanidine Isothiocyanate, Disodium
ethylenediamine tetraacetate.
Storage and Stability
Store at room temperature. Valid for 24 months.
Production date, expiry date: See packaging label.
Specimen Requirements
Human oropharyngeal swab, nasopharyngeal swab.
Precautions
1. Please read this manual carefully before the
experiment.
2. Place the tube vertically while putting the swab
into it. The swab should not be rubbed into the bottle
mouth, so as to avoid the liquid infiltration and
pollution.
3.Dispose of the used swab rod as a contaminant.
4. No antibiotic was used within 3 months before
sampling (if any, please indicate the time and type of
antibiotic).
5. Do not eat, drink, smoke or chew gum within half
an hour before sampling.
6. Clean the mouth by drinking water within half an
hour before sampling.
7. This specimen preservation solutions is only
suitable for acidic or neutral environments extraction
reagents and is not suitable for detection through the
direct lysis and amplification methods (one-step
method).
8. Avoid contact with the skin or mucous membrane.
If contact occurs, immediately flush with water.
Limitations of Procedure
Samples treated with this reagent can only be used
for nucleic acid extraction and detection, not for
antigen detection. This reagent is not suitable for
untreated whole blood and tissue samples.
FAQ:
1. Are you manufacturer?
Yes we are, and we can offer the best price for you.
2. Payment Terms:
We can accept 30% advance and before ship the goods to port pay the balance.
3. How about the quality?
We can offer the samples to you with free, you can check the quality before place the order.
4. How do you treat quality complaint?
First of all, our quality control will reduce the quality problem to near zero. If there is a quality problem caused by us, we will send you free goods for replacement or refund your loss.
5. Could you offer a free sample for the quality test?
Free sample is available for customers.
Company introduction:
Guangzhou DARUI Biotechnology Co.,Ltd is one of the earliest high-tech enterprise oriented in molecular diagnostic techniques. It was listed on the National Equities Exchange and Quotations on July 9, 2015. The headquarters of DARUI is located in Science Park, Guangzhou, China. It consists of scientific research center which covers an area of 7300m2, and class 10,000 and 100,000 clean manufacturing workshops which covers an area of 1200m2. The base is equipped with various advanced scientific research instruments and production facilities. It has reached the level of the leading biotech enterprises.
DARUI is consistently committed to R&D, production and service of in-vitro diagnostic products, including time- resolved fluoroimmunoassay (TRF), chemiluminescence immunoassay(CLIA), Next Generation sequencing(NGS), Immune colloidal gold technique (GICT),enzyme linked immunosorbent assay (ELISA), tandem mass spectrum(MS/MS), time-of-flight mass spectrum(TOF), collagen geldropletculture-drug sensitivity test(CD-DST)etc. diagnostic kits and equipments. Now ,it has obtained CFDA-Certificates and CE-Certificates for varieties of diagnostic kits. Taking "Innovative Technology, Quality First, Prime Integrity, and Service Foremost" as the quality guideline, DARUI has successfully acquired the Certificate of ISO13485: 2016 Registration. With 17 years in development, DARUI has an efficient technology and management team of over 500 staff, including more than 200 doctors, graduates and scientists.As of today, DARUI has set up a distribution network covering all of mainland China.
Guangzhou Darui Biotechnology Co., Ltd. is a subsidiary of DAAN Gene Co., Ltd. of Sun Yat-sen University. DAAN Gene Co., Ltd. of Sun Yat-sen University was established in 1993 and was listed on the Shenzhen Stock Exchange on August 9, 2004.now DAAN GENE is the no-doubt leader in many market it is involved. it's market value is over 1.2 Billion USD. DAAN Gene is a high-tech enterprise of biology with four research institutes and seven holding companies. With the Certificate of GMP/ISO13485, DAAN Gene is consistently committed to R&D, production and service of in-vitro diagnostic products, including qPCR diagnostic kits and equipments. DAAN Gene has obtained CFDA/CE/CMD/CAS certificates and set up a distribution network covering all of mainland China, and the products sale scale extends to overseas, including India, America Europe and some Southeast Asian countries.




FAQ:
1. Are you manufacturer?
Yes we are, and we can offer the best price for you.
2. Payment Terms:
We can accept 30% advance and before ship the goods to port pay the balance.
3. How about the quality?
We can offer the samples to you with free, you can check the quality before place the order.
4. How do you treat quality complaint?
First of all, our quality control will reduce the quality problem to near zero. If there is a quality problem caused by us, we will send you free goods for replacement or refund your loss.
5. Could you offer a free sample for the quality test?
Free sample is available for customers.
Company introduction:
Guangzhou DARUI Biotechnology Co.,Ltd is one of the earliest high-tech enterprise oriented in molecular diagnostic techniques. It was listed on the National Equities Exchange and Quotations on July 9, 2015. The headquarters of DARUI is located in Science Park, Guangzhou, China. It consists of scientific research center which covers an area of 7300m2, and class 10,000 and 100,000 clean manufacturing workshops which covers an area of 1200m2. The base is equipped with various advanced scientific research instruments and production facilities. It has reached the level of the leading biotech enterprises.
DARUI is consistently committed to R&D, production and service of in-vitro diagnostic products, including time- resolved fluoroimmunoassay (TRF), chemiluminescence immunoassay(CLIA), Next Generation sequencing(NGS), Immune colloidal gold technique (GICT),enzyme linked immunosorbent assay (ELISA), tandem mass spectrum(MS/MS), time-of-flight mass spectrum(TOF), collagen geldropletculture-drug sensitivity test(CD-DST)etc. diagnostic kits and equipments. Now ,it has obtained CFDA-Certificates and CE-Certificates for varieties of diagnostic kits. Taking "Innovative Technology, Quality First, Prime Integrity, and Service Foremost" as the quality guideline, DARUI has successfully acquired the Certificate of ISO13485: 2016 Registration. With 17 years in development, DARUI has an efficient technology and management team of over 500 staff, including more than 200 doctors, graduates and scientists.As of today, DARUI has set up a distribution network covering all of mainland China.
Guangzhou Darui Biotechnology Co., Ltd. is a subsidiary of DAAN Gene Co., Ltd. of Sun Yat-sen University. DAAN Gene Co., Ltd. of Sun Yat-sen University was established in 1993 and was listed on the Shenzhen Stock Exchange on August 9, 2004.now DAAN GENE is the no-doubt leader in many market it is involved. it's market value is over 1.2 Billion USD. DAAN Gene is a high-tech enterprise of biology with four research institutes and seven holding companies. With the Certificate of GMP/ISO13485, DAAN Gene is consistently committed to R&D, production and service of in-vitro diagnostic products, including qPCR diagnostic kits and equipments. DAAN Gene has obtained CFDA/CE/CMD/CAS certificates and set up a distribution network covering all of mainland China, and the products sale scale extends to overseas, including India, America Europe and some Southeast Asian countries.





